Casella UK
Wolseley Rd, Kempston,Bedford MK42 7JY
+44(0)1234844100
info@casellasolutions.com

The type of “suitable sampling medium” mentioned above largely depends on the sampling and analytical method selected for the chemical under investigation but are usually a form of sorbent tube (glass or re-usable metal). The Health and Safety Executive (HSE) in the UK and NIOSH in the US publish guidance known as Methods for the Determination of Hazardous Substances (MDHS)¹ and NIOSH Manual of Analytical Methods (NMAM)² respectively.
Sorbent tubes developed in the 1970s for NIOSH have been established as a reliable sampling method for many of these sampling requirements. A sorbent tube contains a chemically inert material which adsorbs the vapour or gas onto its surface during sampling.
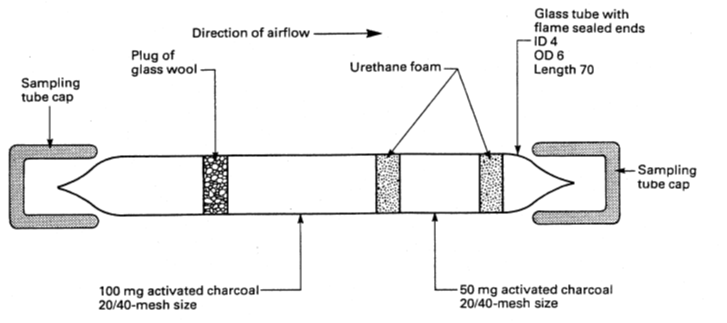
Charcoal is the most common sorbent material but Silica Gel, Chromopak, Tenax and other specialist materials are also used dependent on the method. Most tubes have two layers of material where the smaller layer is the ‘back up’ and should be closest to the sampling pump inlet; there is normally an arrow on the tube which also indicates the correct direction of the airflow. The diagram shows the complexity of the tube which requires material of a specified mesh size and weight in each of the two layers.
Prior to use check that the tubes are ‘in date’ before breaking the ends of the sorbent tube off and inserting them into a tube holder which is then connected to the personal sampling pump using chemically inert plastic tubing such as Tygon. Handle glass tubes with care whilst setting up the sample train and check carefully for leaks. Samples should also be taken with the tube in a near vertical position on the body to prevent ‘channelling’ which could otherwise lead to an underestimate.
After sampling, the tubes are removed and the ends sealed (with the caps provided) and sent off to a laboratory for further analysis along with unused control tubes from the same batch. The sorbent tubes are then desorbed chemically (or thermally in the case of metal tubes) and analysed, generally by gas chromatography (GC). The two layers are analysed separately and if the ‘back up’ layer is more than 10% of the main layer, the tube is deemed to be saturated and another sample must be taken perhaps varying the parameters (time, flow rate, size of tube etc.) to improve the chance of an acceptable sample.
The standard method chosen will recommend a flow rate for sampling and this needs to be set using a suitable flow calibrator pre-sampling and then checked again post sampling. Maintaining a steady flow within +/-5% is vital to ensure that the sampled volume can be calculated from the set flow rate and sample time. A simple rotameter may be used in the field but a digital flowmeter such as the Casella Flow Detective is preferable for its precision and inherent accuracy. Additionally, the recent generation of Casella pumps and calibrator are now equipped with Bluetooth™ connectivity, which means that the whole calibration process can be fully automated thus minimising the risk of set up errors as well as saving the occupational hygienist valuable time. Another benefit of this connectivity is that progress of the sample can be checked remotely using a dedicated phone app (subject to intrinsic safety requirements) without having to disturb the worker thus improving the productivity of the worker and hygienist alike.
In conclusion, like many seemingly routine measurements they still require care and the devil is always in the detail. It is important to check for changes in methods and exposure limits and that the measuring equipment is up to date and regularly serviced. Developments in both pump and flow technology will help to ensure accurate results and improve productivity and will justify overhauling your fleet of pumps.
Reference article: ISO 13137:2013 Workplace atmospheres -- Pumps for personal sampling of chemical and biological agents - Requirements and test methods